
Mercury (chemical symbol Hg, no. 80 in the periodical system of elements, with zinc, cadmium and dysprosium in group 12) is a poisonous metal and thus, is affected by the RoHS directive of the EU [Restriction of (certain) Hazardous Substances in electrical and electronic equipment].
It is liquid at room temperature, but already evaporates slowly then, more quickly at higher temperatures, accordingly. Toxic reactions are shown by the human body after taking in higher doses, for example, of organic compounds of mercury with the food or by breathing in mercury vapour.
Therefore, in production of our discharge lamps we take care especially to reduce the mercury content of the lamps further and further. So since the 1970s, the Hg-content of fluorescent lamps has been lowered by 90%! Dealing with mercury in a responsible fashion, however, also includes recycling of old lamps (WEEE).
Moreover, with electricity generation from fossil fuels mercury is emitted, so that lamps with less energy consumption and long service life will contribute to avoiding mercury even if they contain mercury.
Why do discharge lamps contain mercury?
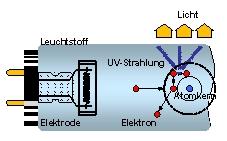
The overall efficiency or luminous efficiency of fluorescent lamps depends mainly on the combination of way of operation, gas filling and kind of phosphor coating.
During operation, the gas discharge within the lamp bulb volume generates invisible UV radiation which is transferred to visible light by the phosphor coating on the inside wall of the lamp bulb. Modern phopshors are tuned in with the UV emission spectrum of the mercury vapour.
So, luminous efficiencies of over 100 lm/W can be reached (with ECG operation). Mercury-free solution can only come up with about 35 lm/W and thus, cannot compete due to their low efficiency.
Visible light in high pressure discharge lamps is generated directly in the light arc by the filling components. Mercury is responsible for the blue part of the spectrum and smooth operation of the lamps.
